| SITE & DATASET | NO (Inc. FU) | ID | DIAGNOSIS | FOLLOW-UP | SCANNER | DATA TYPE | PRIMARY CLINICAL MEASURE |
|---|---|---|---|---|---|---|---|
| KCL_01 | 131 | PSYD_0101 | CHR (56), CHR-FU (30), HC (30), HC_FU (15) | YES | 3T GE | IMG / FS | CAARMS / PANSS |
| KCL_02** | 119 | PSYD_0102 | CHR (85), CHR_FU (2), HC (31), HC_FU (1) | YES | 3T GE | IMG / FS | CAARMS |
| KCL_03 | 58 | PSYD_0103 | FEP (18), CHR (18), HC (22) | 1.5T GE | IMG / FS | CAARMS/ PANSS |
|
| KCL_04 | 26 | PSYD_0104 | SZT (26) | 3T GE | IMG / FS | OLIFE | |
| KCL_05 | 47 | PSYD_0105 | HC (15), SCZ (16), Psychotic Symptoms (16) | 3T GE | IMG / FS | PANSS | |
| KCL_06 | 144 | PSYD_0106 | HC (31), HC_FU (5), SCZ (66), SCZ_FU (42) | 1.5T GE | FS | PANSS | |
| KCL_08 | 38 | PSYD_0108 | SCZ (19), SCZ_FU (19) | 3T GE | FS | PANSS | |
| KCL_09 | 43 | PSYD_0109 | SCZ (30), HC (13) | GE | FS | PANSS | |
| KCL_10 | 37 | PSYD_0110 | FEP (18), HC (19) | 3T GE | IMG / FS | PANSS | |
| KCL_11 | 25 | PSYD_0111 | CHR (8), FEP (12), HC (5) | 1.5T GE | FS | PANSS | |
| KCL_12 | 142 | PSYD_0112 | BPD (41), BPD-R (51), HC (50) | GE | FS | TBC | |
| KCL_13 | 6 | PSYD_0113 | CHR (6) | FU for KCL_02 | 3T GE | IMG / FS | CAARMS |
| KCL_14 | 47 | PSYD_0114 | CHR (45), CHR_FU (2) | FU for KCL_02 | 3T GE | IMG / FS | CAARMS |
| KCL_15 | 12 | PSYD_0115 | SCZ (12) | 3T GE | IMG / FS | CAARMS | |
| KCL_17 | 28 | PSYD_0117 | SCZ (28) | 3T GE | IMG / FS | PANSS | |
| KCL_18 | 57 | PSYD_0118 | SCZ (28), ASPD (13), HC (15) | 3T GE | FS | PANSS | |
| KCL_19 | 45 | PSYD_0119 | SZT (45) | 3T GE | IMG / FS | SPQ / O-LIFE | |
| KCL_20 | 304 | PSYD_0120 | FEP (117), FEP-FU1 (58), FEP-FU2 (35), HC (94) | YES | 3T GE | IMG / FS | PANSS |
| KCL_21 | 291 | PSYD_0121 | FEP (99), FEP-FU (51), HC (95), HC-FU (46) | YES | 1.5T GE | IMG / FS | NO |
| KCL_22 | 44 | PSYD_0122 | HC (8), SCZ (21), SZA (1), SCZ-FU (12), SZA-FU(2) | YES | 3T GE | IMG / FS | PANSS |
| KCL_23 | 30 | PSYD_0123 | CHR(30) | NO | 3T GE | IMG / FS | CAARMS |
| BIRMINGHAM_01 | 42 | PSYD_0301 | CHR (12), FEP (7), ROD(7), HC(16) | 3T Philips | IMG / FS | PANSS / SIPS-P PANSS |
|
| BIRMINGHAM_02 | 28 | PSYD_0302 | SCZ (18), SCZ-FU (10) | 3T Philips | IMG / FS | BAVQ-R | |
| CAMBRIDGE_01 | 40 | PSYD_0401 | SCZ (20), HC (20) | 3T Siemens | IMG / FS | PANSS | |
| CAMBRIDGE_02 | 107 | PSYD_0402 | FEP (22), CHR (34), HC (51) | 3T Siemens | IMG / FS | PANSS | |
| CAMBRIDGE_03 | 66 | PSYD_0403 | SCZ (21), DEP (24), HC (21) | 3T Siemens | IMG / FS | PANSS | |
| NIMHANS_01 | 81 | PSYD_0601 | SCZ (81) | 3T Siemens | IMG / FS | PANSS | |
| NIMHANS_02 | 59 | PSYD_0602 | SCZ (59) | 3T Siemens | IMG / FS | SAP / SANS | |
| NIMHANS_03 | 25 | PSYD_0603 | SCZ (25) | 3T Siemens | IMG / FS | PANSS | |
| NIMHANS_04 | 24 | PSYD_0604 | SCZ (24) | 3T Siemens | IMG / FS | SAP / SANS | |
| NIMHANS_05 | 45 | PSYD_0605 | SCZ (45) | 3T Siemens | IMG / FS | SAP / SANS | |
| EDINBURGH_01 | 92 | PSYD_0701 | SCZ (34), BPD (18), HC (40) | 3T Siemens | IMG / FS | PANSS | |
| CUBIC_01 | 58 | PSYD_0801 | CT (58) | 3T Siemens | IMG / FS | CTQ | |
| CUBIC_02 | 53 | PSYD_0802 | SZT (58), nb.LSZT (26), LSZT (27) | 3T Siemens | IMG / FS | SPQ | |
| CUBIC_03 | 22 | PSYD_0803 | SZT (22) | 3T Siemens | IMG / FS | SPQ | |
| LUBECK_01 | 194 | PSYD_1101 | FEP (77), CHR (73), HC (44) | 3T Siemens | FS | BPRS | |
| JOHN_HOPKINS_01*** | 189 | PSYD_1601 | HC (93), SCZ(50), BPD (21), SA (10), MDD (5), SF (3), Others (5), Substance_induced (2) | NO | Philips MRI | IMG / FS | SCID, SANS / SAPS |
| LMU_MUNICH_01 | 398 | PSYD_1801 | HC (190), BPD (15), BriefPD/CHR (8), DD (2), DIP (9), MD (23), SSD (4), SCZ (105), SZA (42) | NO | 3T Siemens | IMG / FS | PANSS / BACS |
| NAPLES_01 | 104 | PSYD_1901 | SCZ (49), HC (55) | NO | 3T Siemens | IMG / FS | PANSS |
| TOYAMA_01 | 215 | PSYD_2101 | SCZ (77), CHR (51), HC (87) | NO | 3T Siemens | IMG / FS | PANSS / CAARMS |
| MEXICO_01 | 99 | PSYD_2201 | FEP (49), HC (50) | NO | 3T Siemens | IMG / FS | PANSS |
| ZURICH_01 | 119 | PSYD_2401 | HC (79), CHR (10), DD (3), SCZ (22), SCA (5) | NO | 3T Philips | IMG / FS | OLIFE / MSS / PANSS |
| GEORGIA_01 | 143 | PSYD_2501 | SCZ (60), HC (83) | NO | 3T Siemens | IMG / FS | QOL / UPSA |
| SINGAPORE_01 | 79 | PSYD_2601 | CT (39), HC (40) | NO | 3T Siemens | IMG / FS | PQB / CTQ |
| CNP(UCLA)_01 | 265 | PSYD_2801 | SCZ (50), BPD (49), ADHD (41), HC (125) | NO | 3T Siemens Trio | IMG / FS | SAP / SANS, BPRS, SCID |
| FIDMAG(ESP)_01 | 71 | PSYD_3001 | SCZ (46), HC (25) | NO | 3T Philips | IMG / FS | PSYRATS |
| VALLADOLID(ESP)_01 | 295 | PSYD_3101 | SCZ (71), SCZ_FU(6), FEP (87), FEP_FU(12), HC (119) | YES | 3T Philips | IMG / FS | PANSS, BNSS, IPASE |
| SEVILLA(ESP)_01 | 665 | PSYD_3201 | HC(78), SCZ(79), SAD(3), BPD(11), HC_FU(189), SCZ_FU(163), SAD_FU(9), BPD_FU(20), Others(113) | YES | 1.5T GE | FS | SAPS, SANS, IPASE |
| SEVILLA(ESP)_02 | 628 | PSYD_3202 | HC(115), SCZ(77), SAD(3), BPD(37), HC_FU(134), SCZ_FU(69), SAD_FU(4), BPD_FU(31), Others(158) | YES | 3T Philips | FS | SAPS, SANS |
| BERLIN_01 | 222 | PSYD_3401 | HC(49), FEP (17), CHR-P(119), CHR-N(37) | NO | 3T Siemens | IMG / FS | PANSS |
| SICHUAN_01 | 182 | PSYD_3501 | HC (110), FES (72) | NO | 3T GE | FS | PANSS |
| AMSTERDAM_01 | 78 | PSYD_3601 | HC(20), FEP (58) | NO | 3T Philips | IMG / FS | PANSS |
| TOTAL | 6362 |
* = Datasets where a data
shraing agreement is in place but sample size cannot be confirmed
** = Datasets
where follow-up data has a different PSYD code
IMG = Image data
availible, FS = FreeSufer data Availible
*** = Additional conditions may
apply regarding the use of this dataset, please enquire with the Psy-ShareD Team
Memorandum of Understanding (MoU) for Data Access
The Data Access process is governed by a Memorandum of Understanding (MoU), ensuring that all parties agree on the terms of data use, security, and privacy. The MoU defines the roles and responsibilities of the data provider and the requesting party. All users must agree to the MoU before any data can be accessed. See - MoU Documents.
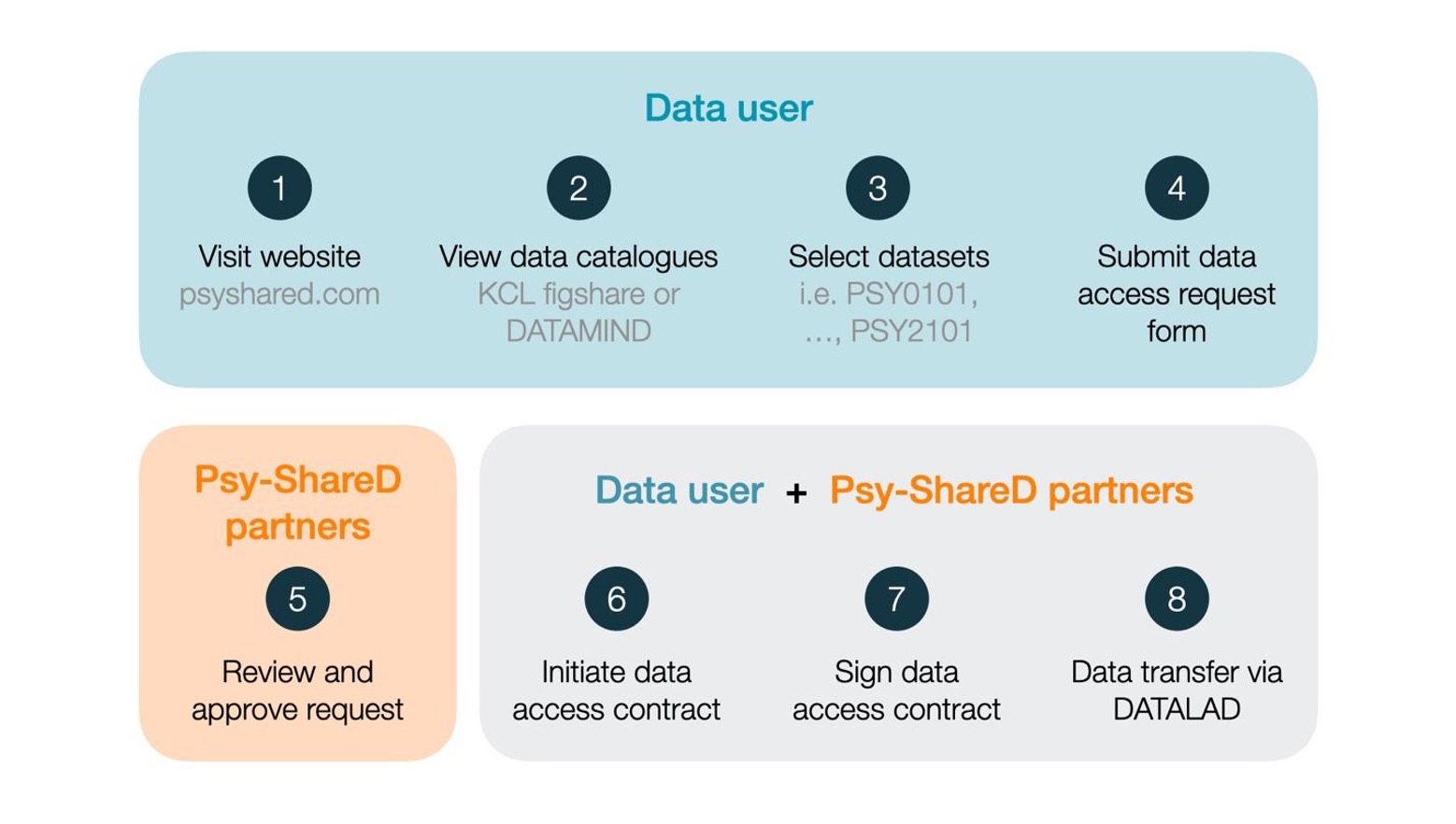
Data Access Procedure
The data access procedure
involves several steps to ensure compliance with data protection policies and fair use
practices. Upon submission of the Data Access Request form, applicants should anticipate a
processing period of several weeks, contingent upon the institution’s administrative
procedures. It is advisable to account for this timeframe in your planning. For a detailed
overview of the process, please refer to the figure below:
Terms and Conditions of Use
The Psy-ShareD data
access procedure
should be followed as illustrated in the figure located in the Data Access Procedure
section. Data catalogues can be viewed on KCL FigShare and via the Psy-ShareD
website. Please note that the variables listed in the data
catalogues are not exhaustive and some datasets may contain variables
not listed in the data catalogues. The data catalogues do list all
primary clinical, medication, demographic and
neuropsychological/cognitive variables.
All data access is via a data access request that is reviewed by the
Psy-ShareD Data Management Committee (DMC). Members of the DMC can be
viewed here. The Data Access Request Form (DARF) can be accessed here.
The DMC will review the data access request (usually within 2-weeks)
and may ask the applicants to resubmit an amended version of the DARF if
clarification is needed (see guidance).
Once a
data access request has been approved by the DMC the Chair will request a
Data Use Agreement (DUA). The DUA is a legal contract that is
established between King’s College London and the recipient institution.
The DUA agreement sets out the terms and conditions of data use as well
as the agreed duration for data use. Data transfer from KCL to the
recipient institution cannot take place until the DUA has been
completed. Due to the time it takes to set up a DUA, Psy-ShareD data may
not be suitable for projects which are working to strict timelines, such as undergraduate
and MSc dissertations
Once the data user(s) have completed their
data analysis any subsequent publications are subject to the publication
policy explained in the Psy-ShareD Memorandum of Understanding (here).
Other terms and conditions that should be noted:
Participant
consent for data sharing and reuse are checked in each dataset. Where
consent for reuse has been withdrawn and not provided individual data
are removed.
In some older KCL datasets information
regarding informed consent is no longer available. However, as these are
fully anonymised datasets their reuse is allowed under NHS Database
ethical approval.
Datasets acquired after July 31st 2022
will need to comply with NHS National Data-Opt out service
(https://digital.nhs.uk/services/national-data-opt-out/compliance-with-the-national-data-opt-out).
Currently Psy-ShareD hosts no datasets that were acquired after this
date.
Healthy Control (HC) data is provided in several
Psy-ShareD datasets. It should be noted by Data users that in some cases
HC were recruited to studies without a full diagnostic assessment for
psychiatric and/or neurological conditions. Data users are responsible for assessing the
suitability of HC control data for their particular studies and hypotheses.
Whist we have worked hard to ensure that all datasets are catalogued correctly we strongly
advise data users to contact and collaborate closely with dataset PIs to ensure the accuracy
of clinical, demographic, and cognitive data.
Data Access Form
To access the data, please fill out the following request form and send it to psy_shared@kcl.ac.uk. Once submitted, your request will be reviewed, and further instructions will be provided.
| Abbreviation | Definition |
|---|---|
| SCZ | Schizophrenia |
| TRS | Treatment Resistant Schizophrenia |
| FEP | First episode psychosis |
| FHR | Familial high risk (FHR) for psychosis |
| SZT | Schizotypy |
| BPD | Bipolar disorder |
| CT | Experience of childhood trauma |
| HC | Healthy controls |
| Psychosis / Functioning | |
| PANSS | Positive and negative symptom scale |
| SAP/SANS | Scales for assessments of positive/negative symptoms |
| CAARMS | Comprehensive assessment of at-risk mental state |
| PSYRATS | Psychosis Rating Scales |
| GAF | General assessment of functioning |
| FIGS | Functioning scale in psychosis |
| PQ-B | The Prodromal Questionnaire-Brief version |
| Depression / Anxiety | |
| HAM- A | Hamilton anxiety |
| HAM-D | Hamilton depression |
| DASS | Depression, anxiety, stress scale |
| BDI | Beck depression inventory |
| Personality | |
| O-LIFE | Oxford and Liverpool inventory for feeling and experience |
| SPQ | Schizotypal personality scale |
| EPQ | Eysenck Personality scale |
| Trauma | |
| CTQ | Childhood trauma questionnaire |
| Cognition / IQ | |
| NART IQ | National Adult Reading Test (IQ estimate) |
| WRAT IQ | Wide Range Achievement Test (IQ estimate) |
| WASI IQ | Wechsler Abbreviated Scale of Intelligence |
| WAIS (digit span, cognitive processing speed) VF | WAIS Verbal Fluency (executive function) |
| WCST | Wisconsin Card Sort Test (executive function) |
| HVLT | Hopkins Verbal learning test (verbal leraning) |
Guidance for preparing a Data Access Requests
The Psy-ShareD Data Management Committee (DMC) provides the following guidance for anyone making a data access request.
- Make sure all sections of the Data Access Request Form (DARF) are completed properly. Any uncompleted sections will result in the DARF being returned without full review and delay the application process.
- Data access applications must be led by a member of staff at a given institution and cannot be led by undergraduate or postgraduate students. Students can be listed as co-investigators.
- Hypotheses and analyses should be focused rather than general and non-specific.
- Applicants should think carefully about which datasets they are requesting access to. There should be clear justification for each dataset requested i.e. a rationale for the use of the dataset(s) in the context of the study hypotheses etc.
- DARFs that list several (or all) datasets should provide clear justification as to
why these are needed in the context of study/hypotheses.
- Timescales should be realistic. Projects that require extensions will need to
contact the DMC and request an amendment to the Data Use Agreement.
- A large number of analysis and being done in parallel with Psy-ShareD data, therefore it is essential that analyses stay within the bounds of their approved data access request form to avoid overlap between studies. It is particularly important that variables that have been listed as covariates are not changed to variables of interest. However, it is also understood that the focus of projects may vary slightly over time; if this is the case the applicant should contact the Psy-ShareD DMC to gain approval for new analyses. It would not be acceptable for example, to present a new analysis beyond the bounds of the synopsis in a draft of the paper before approval is given. In such instances, the Psy-ShareD DMC are likely to withhold approval for the publication of the paper.
Publication Policy
People involved in the primary design, analysis and writing parts of the project/manuscript (usually the Data User(s)) will be listed as first and/or senior (last) authors. In addition, all Psy-ShareD Partners who are site PIs and Co-Is for datasets that have been used in each publication will be listed on the primary author line. The Psy-ShareD Partnership (rather than all individual partners) will also be listed on the primary author line. The Psy-ShareD Partnership will be linked to an expanded author list published within the paper and/or a URL containing all partner information. These conditions are mandatory.
For the full publication policy, see the Publication Policy section of the MoU.
